The first ionisation energy of Li is 5.4 eV and electron affinity of Cl is 3.61 eV. What is the value of Δ H(in kJ/mol) for the following reaction? Li( g) + Cl(g)→

The first ionisation energy of Li is 5.4 eV and electron affinity of Cl is 3.61 eV. What is the value of Δ H(in kJ/mol) for the following reaction? Li( g) + Cl(g)→

Equivalences et mesures de cuisine poids et mesures contenance cuil... | Conversion cuisine, Cuisine, Recette

Calculate the enthalpy change for the process CCl4(g) → C(g) + 4Cl(g) and calculate bond.... - YouTube

Le grand-père, ou, Les deux âges . ^^^^ g^— ^?=^-:=4 l^^^EE^ J^ m ^ p ^^^ fe^^^ Ts ic me siUMi W- ^m m^^m ^^s 1 (• cl IC ^^ ^^

L'Académie du Goût - 6 sauces incontournables de la cuisine française, à garder avec soi. Elles accompagnent viandes, légumes et poissons et peuvent aussi servir de base pour un plat. ✨ Encore
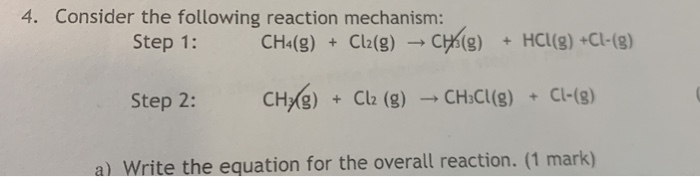
The reaction CH4(g) +Cl2---> CH3Cl(g) + HCl (g) has ∆H = -25kcal and bond dissociation energy of C-Cl, H-Cl, C-H and Cl-Cl is given as 84 kcal/mol, 103 kcal/mol, x kcal/mol and
16. Calculate the enthalpy change for the process CCl4(g)————C(g)+4Cl(g) And calculate bond enthalpy of C Cl in CCl4(g). Δ vapH(CCl4)=30.5 kj /mol Δ fH(CCl4)= 135.5 kj/mol Δ aH(C)=715.0 kj/mol Δ aH(Cl2)=242 kj/mol

Calculate the enthalpy change for the process CCl4(g)→ C(g) + 4Cl(g) and calculate bond enthalpy of C - Cl in CCl4(g) Δ vapH^ (CCl4) = 30.5 kJ mol ^-1 . Δ fH^ (